You may not have asked yourself, "Is this protein its brother's keeper?" but this question is of keen interest to protein biochemists and it formed the background of Rhesa's project.
A living cell is a very crowded place; molecules of various kinds collide, brush past and ram into each other, driven by thermal energy. In this kind of environment, proteins- the workhorses of living cells - sometimes become entangled or badly distorted and dire consequences may ensue.
Diseases, including Alzheimer's disease, Sickle cell anaemia and Huntington's disease, are associated with protein distortion, called "misfolding". Misfolding is often followed by inappropriate associations of proteins to form "plaques" piles of molecular junk that can be toxic to the cell.
Fortunately, Nature has come up with a cunning solution: a family of proteins named "chaperones". As with human chaperones, these molecular chaperones keep newly made proteins out of trouble and help them fold into their functional shapes by providing an environment shielded from inappropriate associations with other molecules. One might say that these chaperones keep young proteins from hanging with the wrong crowd.
The 70-kilodalton heat shock protein ("Hsp70" in short) is an important molecular chaperone in organisms from bacteria to humans. Hsp70's role in keeping other proteins out of trouble helps with a wide range of activities, from helping us withstand high temperature to transmission of signals in the brain. In his summer research project, Rhesa set out to investigate just how Hsp70 does its job and, in particular, to study how it moves and flexes in the process.
Proteins are the molecular machines of the cell, copying DNA, moving nutrient about, sensing the outside world, turning genes on and off and much more. To do all these things they must move and flex, just like larger machines.
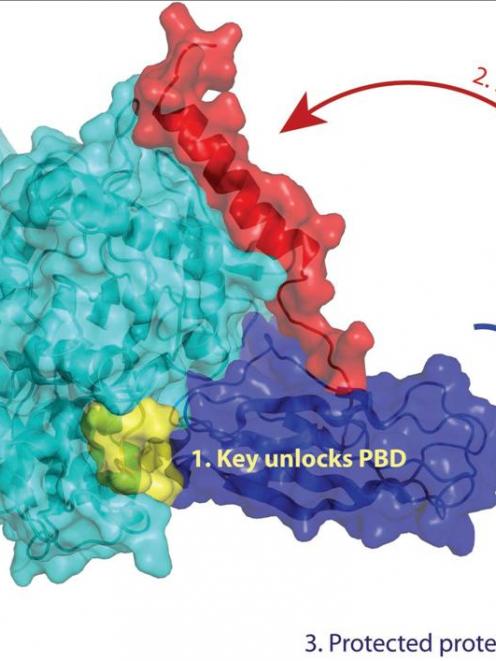
Hsp70 is no exception; it looks a bit like a flexible dumbbell, with two compact ends able to move relative to each other at opposite ends of a bending bar. One end (called the PBD for "protein binding domain") of this molecules acts like a padlock, whose shackle can close around another protein and hold it tight. The other end of Hsp70 acts like a key to open and close the padlock. The key end binds to the energy-rich nucleotide ATP and so is called the NBD. Only with ATP bound does the NBD unlock the PBD.
Working with Dr Sigurd Wilbanks of the Biochemistry Department, Rhesa studied how the two ends meet one another. From earlier work they already knew that the two ends come together, but it was unclear how the key fit into the lock or what moved as Hsp70 became unlocked.
Many techniques for looking at the three-dimensional structure of proteins, for instance electron microscopy and X-ray crystallography, show only a snapshot, but tell little about how the protein moves. More dynamic techniques like spectroscopy, which measures the absorption or fluorescence of light, can give clues about motion.
Rhesa used the transfer of photons of light from one part of the molecule to another to ask which parts are close together. This is like heating one part of the key and discovering that only a small part of the lock warms up in response. One can conclude these two parts must be close together.
Making several of such measurements for different parts of the PBD allowed the team to draw a model of how the NBD fits into the PBD and unlocks it, just as a surveyor uses multiple measurements to compose a map. This picture of Hsp70 in action sheds light on how proteins physically move to perform their functions in the crowded fast-paced world of the cell.
- Dr Sigurd Wilbanks, Lecturer, Department of Biochemistry and Rhesa Buhidarmo, BSc (Hons) student
